Our proprietary portfolio includes NBD1 stabilizers and complementary modulators. We believe the synergistic approach of combining NBD1 stabilizers with complementary modulators provides the highest probability of normalizing CFTR function for CF patients. Our programs target the NBD1, ICL4, and TMD1 regions of CFTR.
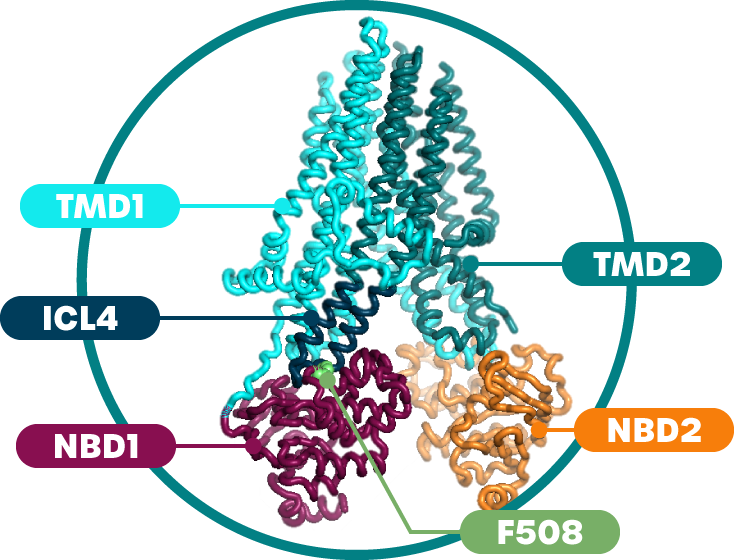
ICL4 – Intracellular Loop 4 of CFTR, NBD1 – Nucleotide Binding Domain 1 of CFTR, TMD1 – Transmembrane Domain 1 of CFTR.