Redefining what’s possible in cystic fibrosis treatment
Our vision is to restore CFTR function for people living with CF.

Who We Are
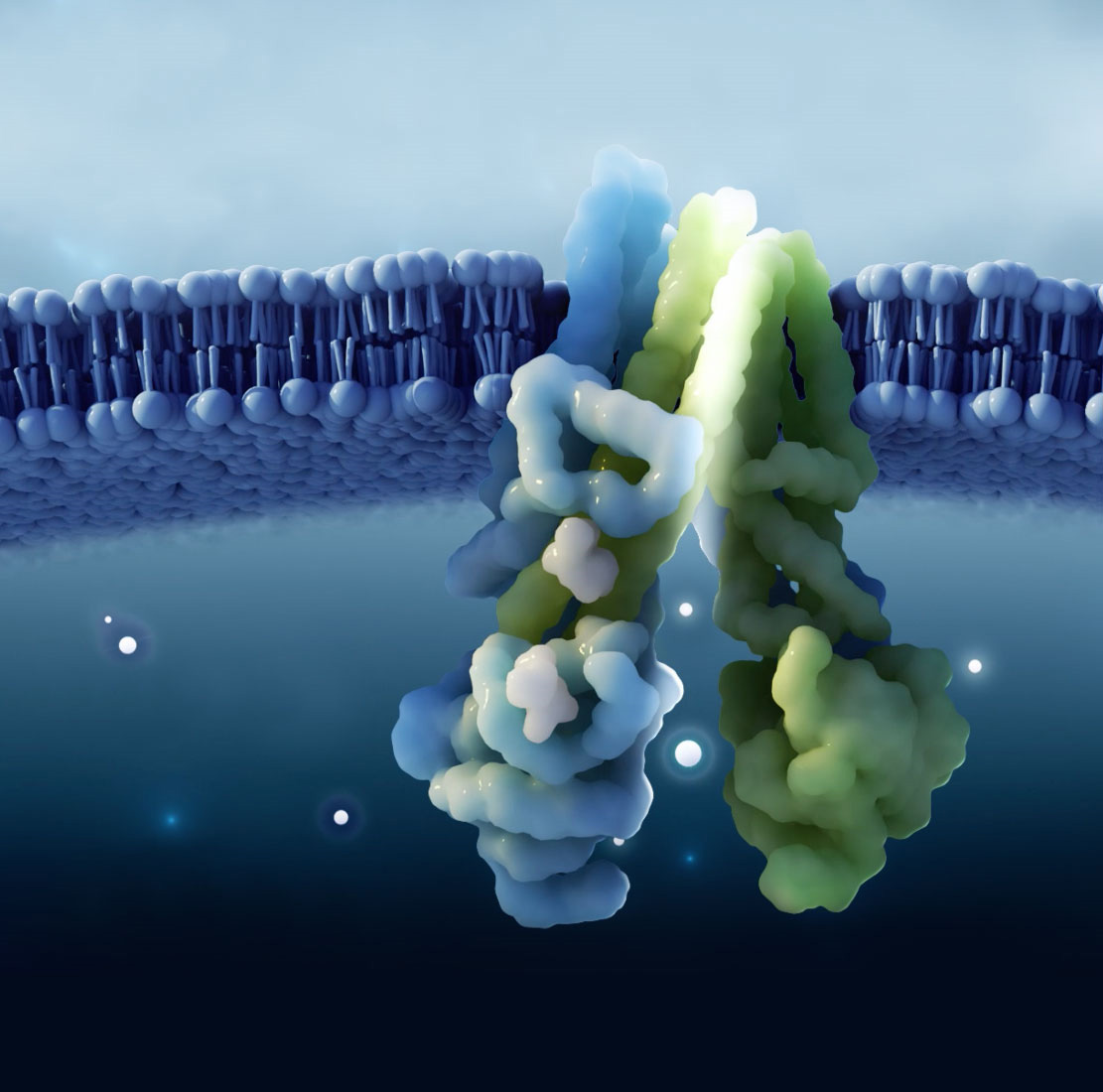
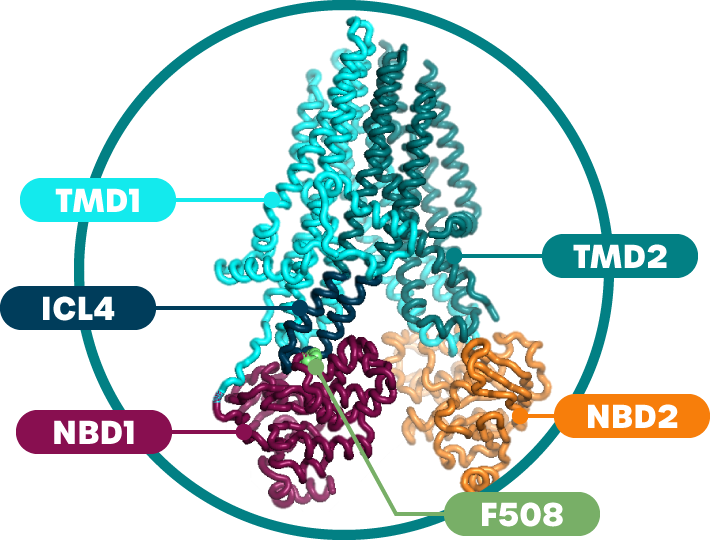
Sionna Therapeutics is a clinical-stage biopharmaceutical company on a mission to revolutionize the current treatment paradigm for cystic fibrosis (“CF”) patients by developing novel medicines that normalize the function of the cystic fibrosis transmembrane conductance regulator (“CFTR”) protein to deliver clinically meaningful benefit to CF patients. Our goal is to deliver differentiated medicines for people living with CF that can restore CFTR function to as close to normal as possible by directly stabilizing CFTR’s nucleotide-binding domain 1 (“NBD1”). Leveraging more than a decade of our co-founders’ research on NBD1, we are advancing a pipeline of small molecules engineered to correct the defects caused by F508del, the most common CF genetic mutation, which resides in NBD1. We are also developing a portfolio of complementary CFTR modulators designed to work synergistically with our NBD1 stabilizers to improve CFTR function.
Our vision is to build a CF franchise anchored by our NBD1 stabilizers to deliver clinically meaningful benefit to people with CF.